In the cultivation of both Anthurium and Phalaenopsis, it is important to continuously monitor the cultivation. Growers strive to keep cultivation conditions constant throughout the year. Unfortunately, they have to deal with many different external influences. Besides insects, fungi and bacteria, inaccuracies in pH/EC, nutrient composition, starting water, etc. can have a major impact on the crop. Current research techniques allow for the timely detection of threats. Recognizing a threat early is crucial to preventing or limiting crop damage.
In this article the different analysis methods and their application in practice are discussed:
- Chemical water analysis
- Microbiological water analysis
- DNA Multiscan® technique
- Heavy metal analysis
- Othere analyses
The attached diagram shows where each method can be used on your farm.
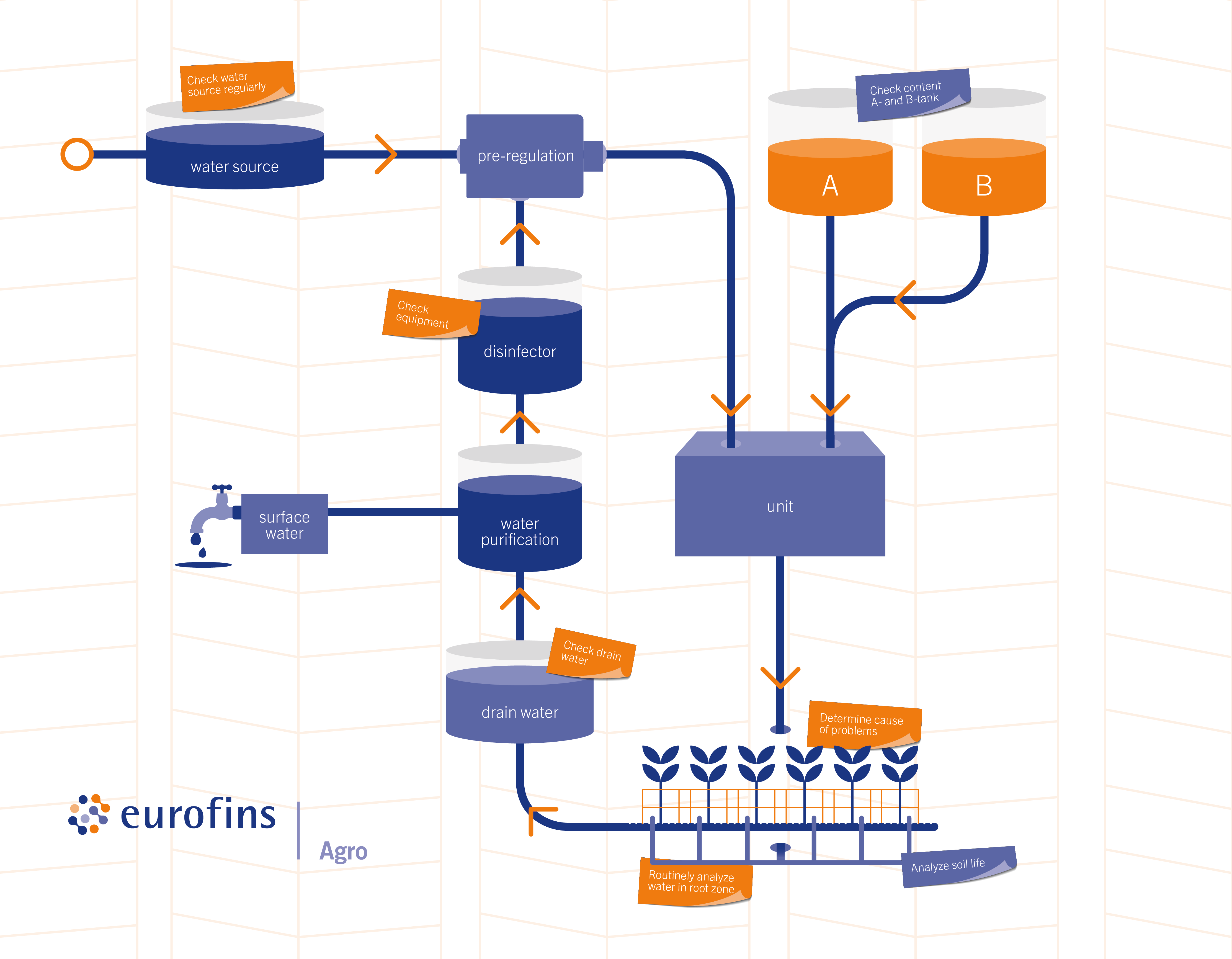
Test locations greenhouse (source: https://www.eurofins-agro.com/)
1.Chemical water analysis
Good quality water is essential for cultivation. Several water analyses can be performed to check the water quality.
Starting water
The starting water should be as pure as possible; it should not contain too many salts or nutrients. Rainwater is ideal as starting water. If there is insufficient rainwater available, well water, possibly treated with reverse osmosis, can also be used. The disadvantage of well water is that its composition is not always constant: the hardness of the water, the amount of salts, and possible contamination can change the longer the well is in use. Reverse osmosis can go some way to moderating this problem; however, osmosis filters may allow more elements to pass through as they age. The pH of the starting water also affects the efficiency of osmosis filters. Regular maintenance and monitoring can prevent reverse osmosis problems.
Rainwater is generally very stable in quality, but there are a number of factors that influence the quality of rainwater. If the chalk has just been applied, or if the chalk is removed, there will temporarily be more calcium in the rainwater. This causes an increase in calcium and a rise in pH.
Irrigation water
The composition of irrigation water can change, due, among other things, to the starting water, the composition and dosage of the manure tanks, and micro-organisms in the basins, pipes and substrate. When recirculation takes place, the composition of the drain also has an influence.
Conversion by micro-organisms
In the basins, the pipes and the substrate, various micro-organisms can be found that convert the nutrients in the irrigation water. These micro-organisms mainly influence the amount of nitrogen and the pH of the irrigation water. Urea and ammonium are converted into nitrate, lowering the pH of the water. Certainly in the daily stock or in the drain silo these effects are not negligible
The irrigation water is best checked at the outlet from the rain pipe, dripper or floor. This way you measure the water that actually reaches the plant.
> Always measure the pH immediately after taking the sample. The pH of the water changes rapidly and the pH measured by the laboratory is often higher than in reality.
Drainage water
The drainage water is a good way to monitor which nutrients are well absorbed by the plant and which elements are present in excess. During cultivation, the EC will increase due to substrate evaporation and because not all elements are fully absorbed by the plant. This causes salinization of the substrate and can cause damage to the roots. Furthermore, the roots of plants release hydrogen ions during cultivation when cations such as ammonium, calcium, potassium and magnesium are taken up. This causes acidification of the substrate. A drain or squeeze sample can provide insight into the degree of salinization and acidification. A chemical analysis of the drainage water also provides insight into the shortage or surplus of the various elements. When recirculating, it is important to regularly adjust the fertilizer tank filling to the composition of the drainage water.
2.Microbiological water analysis
In addition to the chemical composition of the water, the microbiological composition is also important for the quality of the water. With a colony forming units (CFU) analysis the amount of bacteria and fungi in the water can be determined. This analysis is useful, among other things, for measuring the effectiveness of the disinfector and the number of micro-organisms in the basins. By doing a CFU analysis a few times a year, you can learn more about the development of micro-organisms in the water over a certain period of time. A special procedure applies to the taking of these samples, so that the sample does not become contaminated.
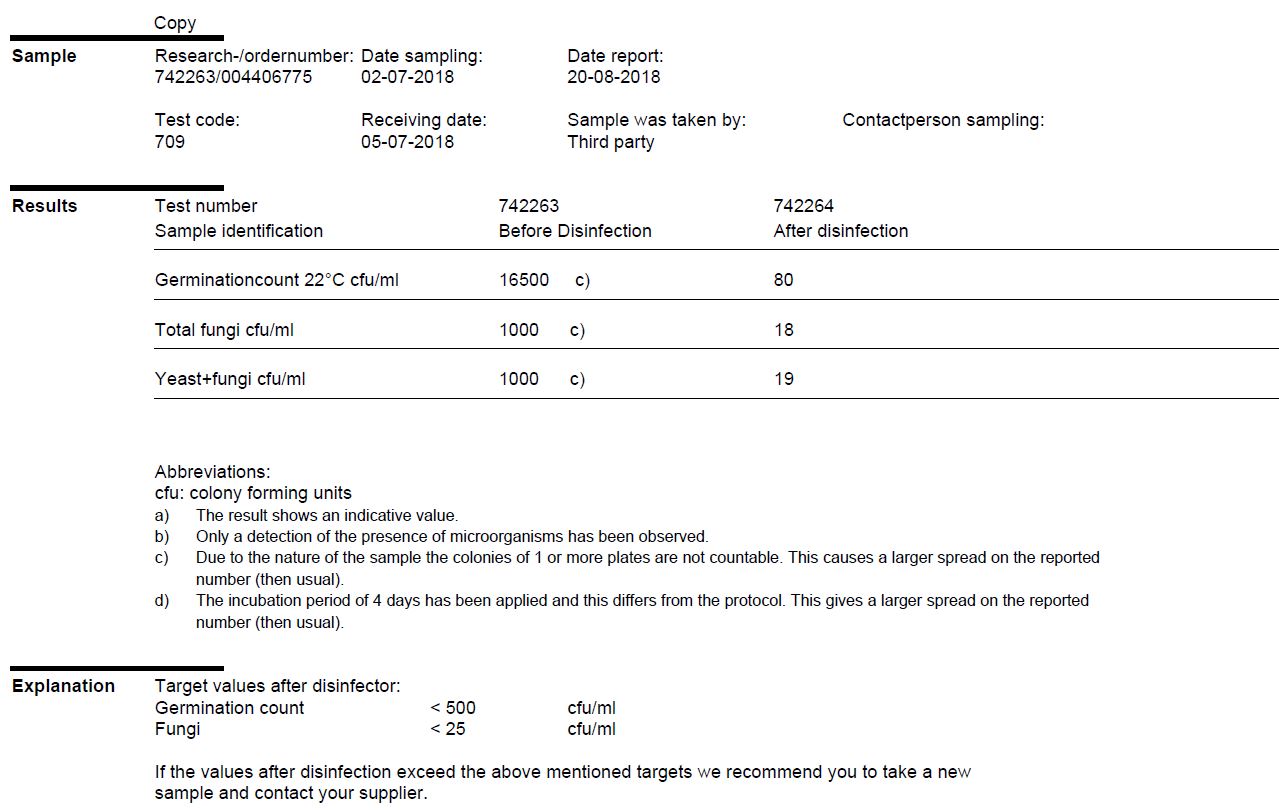
Colony forming units (CFU) analysis: disinfector check
3.DNA Multiscan® technique
The CFU analysis does not provide any insight into the exact micro-organisms present in the water. Not all bacteria or fungi are plant pathogens. A DNA analysis shows which bacteria and/or fungi are present in the water, so that targeted action can be taken. A DNA analysis also shows dead fungi and bacteria and can therefore give a distorted picture when recirculating with a disinfector. Nevertheless, it gives an indication of which micro-organisms are present in the greenhouse. When it appears that too many harmful bacteria are present in the water, various measures can be taken to improve the water quality. These measures are described in the article ‘Healthy water as basis for healthy cultivation’
4.Heavy metal analysis
Heavy metals can occur not only in the water and substrate but also in the cultivation system. When using groundwater, the reverse osmosis filter may allow more boron or arsenic to pass through over time. Changing pH also affects the efficiency of osmosis filters. An excess of boron or arsenic can cause leaf spots in the crop.

Leaf tips in Phalaenopsis caused by an excess of heavy metals.
Depending on the source, the substrate may also contain heavy metal pollutants. In the cultivation system, aluminium or zinc can be released from aluminium roll containers or galvanized wire mesh bases. This can be particularly problematic when the percentage of recirculation water is high. Zinc can compete with iron absorption in orchids, and excess zinc is therefore usually visible as iron deficiency.
When there is a suspicion of poisoning by heavy metals, a heavy metal analysis of the substrate or water can give a decisive answer. A standard chemical analysis only investigates boron and zinc levels; an analysis for aluminium or arsenic has to be requested separately.
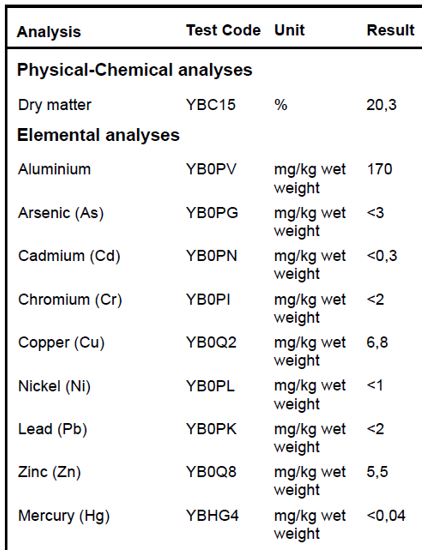
Results of heavy metal analysis.
Other analyses
Virus research
If virus symptoms are visible, it may be good to know what viruses are involved. The presence of viruses can be demonstrated by means of ELISA (a serological test). Many viruses such as INSV, TSWV and CaCV (Capsicum Chlorosis Virus) are transmissible via thrips. It is then best to remove these plants in time. Other orchid viruses are not spread by insects and therefore there are fewer risks.

Virus damage in Phalaenopsis.
Environmental research
Besides heavy metals, other pollutants may also occur in the substrate. This can sometimes cause ‘unexplained’ growth retardation. With an environmental survey, a wide range of possible pollutants can be investigated. This type of survey can reveal mineral oils, residues of plant protection products, heavy metals and inorganic compounds such as cyanides.

Image of damage to an Anthurium leaf.
Rinse sample of insects
Taking a rinse sample has the advantage that it provides reliable information on the numbers of biological control agents and thrips in the crop.
The incoming material is cut into equal sizes. The young plant material is then rinsed with hot water for a fixed period to kill the thrips and predatory mites and to detach them from the young plant material. After rinsing with hot water, several sessions of rinsing with cold water follow in different sinks. Various sieving techniques are used to first remove the coarser parts, leaving only the thrips and predatory mites.
The process ends with a 90 micron sieve with a counting grid underneath. The thrips and predatory mites fall through this sieve and can then be further examined and counted under the microscope.
A rinse sample always yields a higher number of insects than a ‘dry’ counted sample. As several thrips species are hardly visible in the crop, but do cause damage to it, this method is highly recommended for mapping the thrips problem. If the presence of an unknown thrips species is suspected, it is also possible to make a determination on the basis of adult thrips.

Rinsing device for insects (source: Koppert Biological Systems)
Plant juice/dry matter
In a plant juice test, the juice of the plant is examined for nutritional elements. It provides a snapshot of the absorbed nutritional elements in the very short term. It can be a valuable addition to regular studies of soil and drainage water. By comparing young and old leaves, it is possible to identify at an early stage which elements are at risk of deficiency. In the context of improving plant resilience, this method is helpful for closely monitoring nitrogen and phosphate in the plant. This research can also provide additional information in the event of growth problems in the crop. Plant juice samples must be taken according to a strict protocol, otherwise nothing can be deduced from the figures.
A dry matter analysis shows the history of the nutrient build-up in the plant. For Anthurium, especially in terms of calcium, there is a lot of information available. In an investigation of the cause of brown lobes in the bracts, this method can be very valuable.

Brown lobes in a cut Anthurium.
Plating technique (determination of fungi/bacteria)
By using selective media, we can allow specific fungi to develop in the research laboratory. The fungi are examined microscopically in terms of morphology. In addition, DNA techniques can be used to take a closer look.
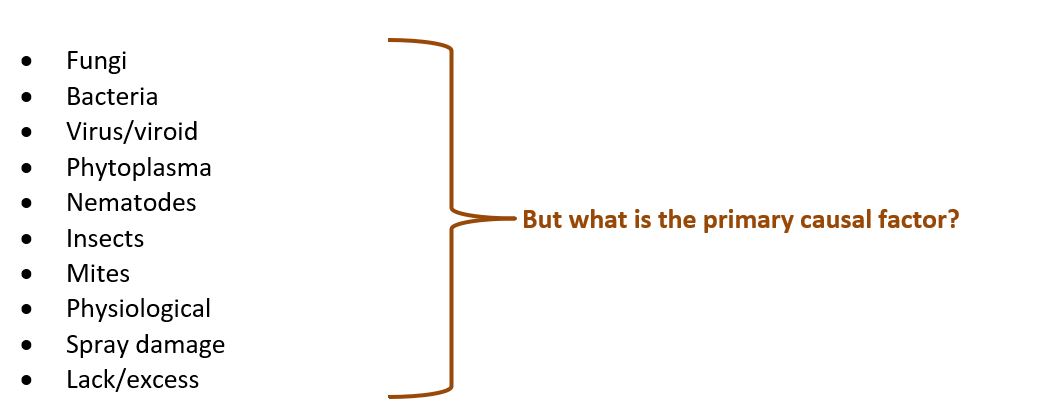
Visual assessment of problems by plant specialists
When you have no idea what a cultivation or growth problem is caused by, a section of a plant can be sent to the laboratory. A team of plant specialists checks the plant visually by microscope, isolating a primary pathogen where necessary and making a pure culture. Additional DNA techniques such as a DNA Multiscan or specific sequence analysis may also be helpful in detecting a possible pathogen. The illustration shows which cases are under investigation.
Soil Life Monitor
With a view to resilient cultivation, the developments in the field of measuring micro-life in the substrate are rapid. By using a PLFA analysis, the micro-organisms in the soil life cycle can be mapped out.
PLFA stands for phospholipid fatty acids. These fatty acids are found in the cell membranes of living organisms. Different groups of organisms have a unique composition of these PLFA fatty acids. By measuring and quantifying PLFAs, a fingerprint of the soil food web can be provided. For example, the cell membranes of fungi consist of different PLFAs than bacteria. The PLFAs present are measured and quantified using gas chromatography-mass spectrometry (GC-MS).
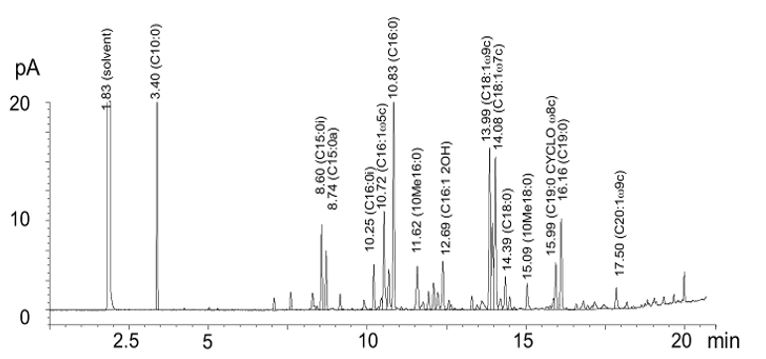
Results of PLFAs measured by gas chromatography (GC-MS).
Organisaties waar onderzoek gedaan kan worden zijn:
Genoemde organisaties hebben ook in het buitenland veelal partners waar ze mee samenwerken en waar een deel van de onderzoeken gedaan kan worden. Wat niet lokaal onderzocht kan worden, kan in de Nederlandse laboratoria worden bekeken.
Met bovenstaande analyses, technieken en tips bent u mogelijk vele bedreigingen voor. Mochten er toch problemen ontstaan, dan reikt dit artikel handvatten om deze zo goed mogelijk op te lossen. Komt u er zelf niet uit, dan kunt u natuurlijk altijd contact opnemen met onze afdeling gewasoptimalisatie.





