When growing Phalaenopsis and Anthurium, insects can sometimes be observed in the greenhouse, both those that are beneficial to the crop and those that are harmful to the plants. A few years ago, we decided to map out which insects were prevalent at our production sites. We tried to identify all the bugs using sticky traps. The disadvantage of sticky traps is that sometimes distortion occurs, so we were not always able to identify the species with certainty.
Identification of thrips
Identifying thrips is difficult because both the different adult thrips and the larvae often look very similar. An example of this is the Echinothrips americanus and Hortensia thrips (Thrips setosus), which can hardly be differentiated at first sight. To distinguish between these thrips, experts are required. However, their availability is very limited in real life. As a matter of fact, very few people have this expertise. Identifying thrips with the help of experts is a lengthy and therefore expensive process. This is unfortunate, because timely identification is extremely important. A new generation can emerge within two weeks, so the earlier it can be determined which insects are present in the crop, the sooner the control can be adjusted accordingly. In addition, each insect, as well as each thrips, has its own way of life.
We soon came to the conclusion that we could not identify the thrips properly with sticky traps; that the different thrips look very similar; and that young larvae cannot be distinguished from each other. Apart from the fact that the diagnosis was difficult and sometimes not reliable enough, it also took too long. Then the question arose: “How can we do this faster and better? With this in mind we set to work.
“With the knowledge we have today, we can conclude that there have been misdiagnoses in the past, which is not surprising given the similarities, but this may have led to incorrect treatment,” explains Marcel van Twist, Research Manager Cultivation.
Research Center
The Anthura Research Centre, opened in 2019, focuses on the development of selection methods, development of marker-assisted breeding, improvement of diagnostics of diseases and pests and optimization of cultivation. Thanks to the knowledge and equipment available in the Research Centre, we were confident that we would be able to develop a test to identify thrips. First, the Cultivation Research Department searched for already published diagnostic protocols. Questions that were raised included: “What do we know already?”, “Have testing protocols been published?”, and “What can we technically implement?” Sufficient information was available for three thrips species: Western flower thrips (Frankliniella occidentalis), Echinothrips (Echinothrips americanus) and onion thrips (Thrips tabaci).
- Western flower thrips
- Echinothrips
- Onion thrips
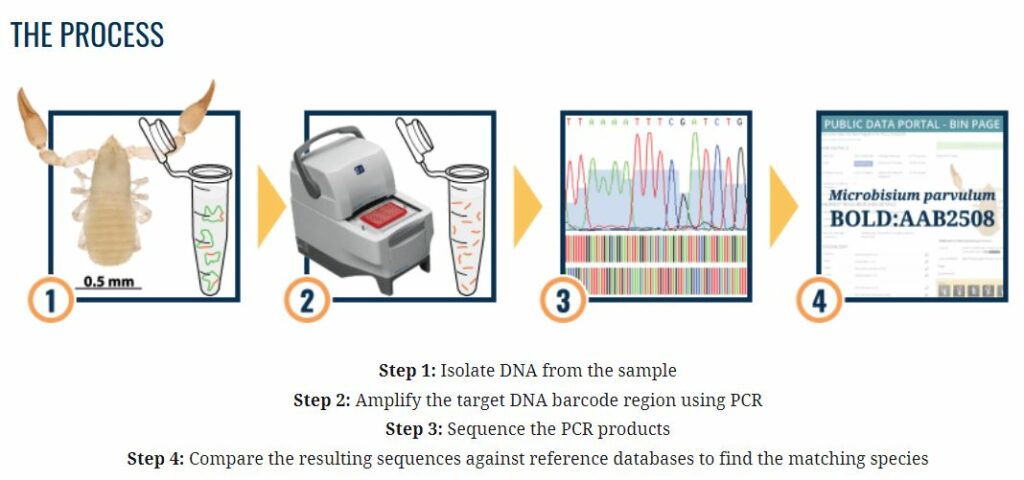
Ruben Vijverberg, Cultivation Researcher, and Marcel van Twist, Cultivation Research Manager, used their knowledge to conclude that identification with a PCR test should be possible. Together with Yvonne de Wit, Head of the Molecular and Diagnostic Laboratory, they searched for the right PCR test to identify the three thrips species with certainty. Once the PCR tests for the three thrips species were in place, the method was further developed, optimized and, above all, accelerated. It was determined how the thrips could be best supplied and how many thrips would be needed to perform the test and make the diagnosis.
Together we learn more
That made us want to know more, because there were (and are) all kinds of other thrips species for which there was no reliable and/or complete literature. Together with Bas Brandwagt, Research Manager Breeding & Phenotyping, we explored whether we could develop PCR tests for tobacco thrips (Thrips parvispinus) and chrysanthemum thrips (Thrips nigropilosus). This involved the use of the ‘tree of life’: a large DNA database of all organisms, including thrips. With the knowledge from previous PCR tests, we succeeded in creating a test for these thrips species, which now allows us to reliably identify tobacco thrips and chrysanthemum thrips as well.
Future
We have now arrived at the thrips species for which there is no or hardly any public information. The current procedure is to first make a classical (visual) identification, then isolate the thrips DNA and see if we can design an appropriate diagnostic test. Of course, we would like to be able to identify all possible thrips species, preferably using PCR tests. This mutual cooperation is highly promising and we can also use it to distinguish other pests.
“Through learning, we see that visual identification has also increased from 30% reliability to 80%”, according to Yvonne de Wit, Head of Molecular and Diagnostic Laboratory.

What are the benefits?
After a year of research, we can now determine without any doubt within three days whether and which thrips – larva or adult – have been caught in a greenhouse and/or are present on the regular sticky traps. This means we can start controlling the same week.
“The identification programme has been running successfully for half a year now and we can proudly say that we can now deal with thrips more effectively”, confirms Peter Vogel, Cultivation Manager Anthurium
For the complete trip recognition map, click on the link below:
https://www.wur.nl/nl/onderzoek-resultaten/onderzoeksinstituten/plant-research/glastuinbouw/show-glas/nieuwe-versie-tripsherkenningskaart-beschikbaar.htm








